by Gopinathan K. Menon and Roger L. McMullen
The Basement membrane zone —also known as the dermal-epidermal junction—lies between the epidermis and the dermis, and functionally is very significant for skin health. Functions of the basement membrane zone include adhesion of the epidermis to the dermis, maintaining the polarity of stratifying epidermis, regulating dermal-epidermal interactions that are significant in development and reparative events, and controlling permeation of substances from the dermal to epidermal compartment. In addition, the basement membrane zone binds a variety of cytokines and growth factors, serving as a reservoir for their controlled release.1 Mutations to the components of the basement membrane zone lead to heritable and blistering skin diseases.
Morphological Components of the Basement Membrane Zone
In histology, the basement membrane zone is identified by Periodic acid-Schiff (PAS) staining as an undulating structure in young/healthy skin, but tends to flatten in aged skin, which is especially evident in photoaged skin due to damage from solar radiation. Ultrastructurally, the basement membrane zone is a 0.5–1.0 μm thick layer that consists of three morphologically distinct divisions: hemidesmosome anchoring filament complex, basement membrane, and anchoring filaments (see Figure 1).
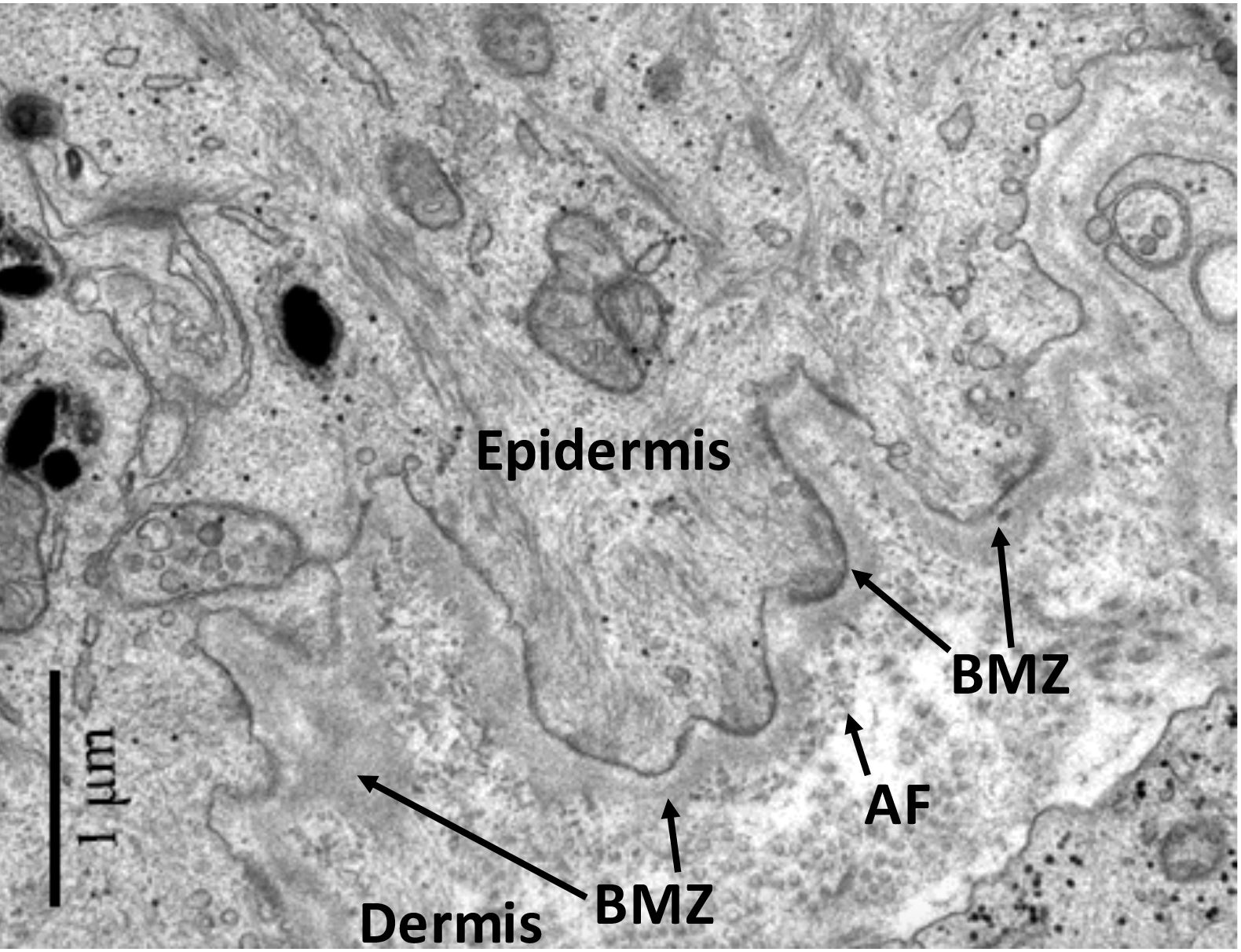
Figure 1: Transmission electron micrograph of
the basement membrane zone of human skin. The location of the epidermis, dermis, basement membrane zone (BMZ), and anchoring filaments (AF) are shown. Image modified from. G.K. Menon, "Skin Basics; Structure and Function" in Lipids and Skin Health, Ed. A. Pappas, Springer: New York (2015).
Hemidesmosomes—from keratinocytes of the basal layer of the epidermis—bind keratinocytes to the basement membrane. Figure 2 contains an illustration of the binding of a hemidesmome to the basement membrane (lamina lucida and lamina densa), which in turn is bound to the dermis by anchoring fibrils.
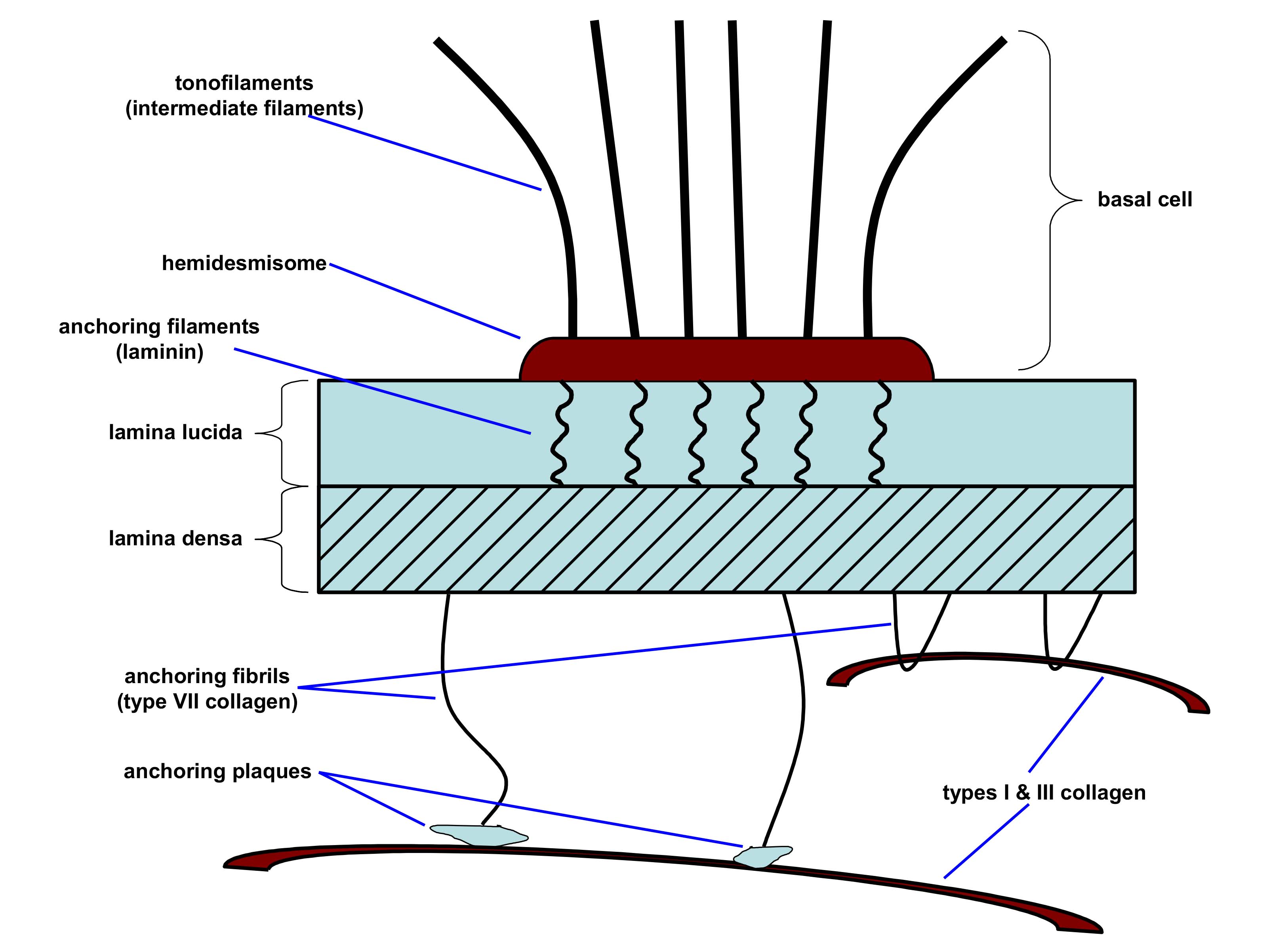
Figure 2: Illustration of the connection of a basal cell keratinocyte with the BMZ and dermis. Adapted from D.T. Woodley and M. Chen, “The basement membrane zone” in The Biology of the Skin, Eds. R.K. Freinkel and D.T. Woodley, The Parthenon Publishing Group: New York (2001), pp. 133-152.
The lamina lucida is an electron lucent (lightly stained) component of the basement membrane composed of laminins, which are heterotrimeric proteins of various combinations of alpha, beta, and gamma protein submits. They are secreted by keratinocytes and serve as fine anchoring fibers, connecting hemidesmosomes with the underlying lamina densa layer. Another constituent of the lamina lucida is fibronectin, which is associated with collagen fibers and plays an important role in wound healing and other biological processes.2 Proteoglycans, such as nidogen and perlecan, bridge these scaffolds by their multiple binding sites for laminin and collagen IV, including the perlecan heparan sulfate chains.3-5
The lamina densa (35-45 nm thick) is an electron dense (heavily stained) layer—also part of the basement membrane—made up mostly of type IV collagen, perlecan (heparan sulfate proteoglycan), and, possibly, laminin. The lamina densa serves as a barrier/filter for the dermis not allowing molecules greater than 40 KDa to pass.
The sub-lamina densa is the region below the lamina densa containing fibrillar structures that connect the lamina densa to the dermis. It consists of anchoring fibrils that attach to collagen fibers by looping around collagen structures and also via connections with dermal plaque-like structures (see Figure 1). These fibrils are primarily composed of type VII collagen, which is secreted both by keratinocytes and fibroblasts.6
| Plectin | A plaque-like protein of the plakin family that mediates attachment of hemidesmosomes to keratin intermediate filaments. Defects in plectin lead to skin fragility. |
|---|---|
α6β4 Integrin |
A transmembrane receptor that promotes cell-cell and cell-matrix adhesion. Several α and β subunits have been identified, and specific α/β chain combinations define cell types. All cells express integrins and specific combinations define cell types. α6β4 Integrin binds to components of the basement membrane zone. Mutations in either chain lead to epidermolysis bullosa. |
Bullous pemphigoid antigen 1 (BPAg1) BP230 |
A glycoprotein member of the plakin family |
Bullous pemphigoid antigen 2 (BPAg2), more commonly known as type XVII collagen |
A transmembrane protein expressed in basal keratinocytes and associated with the hemidesmosome. Patients with mutations in type XVII collagen usually have a form of epidermolysis bullosa. |
Implications of Basement Membrane Zone Damage in Photoaging
In photoaging, alterations in the structure of the basement membrane zone, such as duplication of lamina densa, have been reported.7 These changes result in a more fragile epidermal-dermal interface with weak epidermal resistence to shearing forces in aged skin. Furthermore, it has been reported that increased production of matrix metalloproteinases (MMPs) in photodamaged skin is a major factor in disrupting the lamina densa.8
Concluding Remarks
In closing, the basement membrane zone is an intricate morphological structure that connects the dermis with the epidermis. It is comprised of a variety of structural proteins that give its three zones with distinct characteristics. The basement membrane zone serves as a barrier for the entry of substances from upper layers of the skin as well as a channel for nutrients to pass from vasculature tissue in the dermis to the epidermis. Genetic defects in its structure lead to serious disease states in which the skin’s overall function is compromised. As several anti-aging ingredients—including retinoic acid—have been claimed to improve the basement membrane zone, or some of its specific components, the structure and function of the basement membrane zone should be of interest to the cosmetic chemist.
References
1. R.V. Iozzo, Basement membrane proteoglycans: from cellar to ceiling, Nat. Rev. Mol. Cell Biol., 6, 646-656 (2005).
2. D.F. Mosher and L.T. Furcht, Fibronectin: review of its structure and possible functions, J. Invest. Dermatol., 77, 175-180 (1981).
3. D.T. Behrens, D. Villone, M. Koch, G. Brunner, L. Sorokin, H. Robenek, L. Bruckner-Tuderman, P. Bruckner, and U. Hansen, The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens, J. Biol. Chem., 287, 18700-18709 (2012).
4. D. Breitkreutz, I. Koxholt, K. Thiemann, and R. Nischt, Skin basement membrane: the foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan, Biomed. Res. Int., Article ID 179784 (2013); http://dx.doi.org/10.1155/2013/179784.
5. I. Sher, S. Zisman-Rozen, L. Eliahu, J.M. Whitelock, N. Maas-Szabowski, Y. Yamada, D. Breitkreutz, N.E. Fusenig, E. Arikawa-Hirasawa, R.V. Iozzo, R. Bergman, and D. Ron, Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation, J. Biol. Chem., 281, 5178-5187 (2006).
6. M.P. Marinkovich, D.R. Keene, C.S. Rimberg, and R.E. Burgeson, Cellular origin of the dermal-epidermal basement membrane, Dev. Dynam., 197, 255-267 (1993).
7. R.M. Lavker, Structural alterations in exposed and unexposed aged skin, J. Invest. Dermatol., 73, 59–66 (1979).
8. S. Amano, Possible involvement of basement membrane damage in skin photoaging, J. Invest. Dermatol. Symp. Proc., 14, 2–7 (2009).
RETURN TO STRUCTURE OF SKIN MENU