by Gopinathan K. Menon and Roger L. McMullen
The dermis constitutes the bulk of the skin, accounting for about 90 percent of its total weight. It provides the skin with pliability, tensile strength, and elasticity. The dermis also binds water, protects the body from mechanical injury, and plays an important role in thermal regulation. It is also integral for sensory perception and immune system function.
The dermis contains fibrous, filamentous, and amorphous connective tissue. There is also a vast system of vascular and nerve networks as well as epidermally derived appendages, such as teeth, nails, pilo-sebacious structures, and sudoriferous glands.
The dermis is usually divided into two histologically distinct components. The most superficial layer of the dermis is the papillary dermis. It is located directly below the basement membrane zone and contains a loose network of connective tissue consisting of thin collagen fibers. The deeper dermis—the reticular layer—has a much denser connective tissue matrix. The reticular dermis contains thicker and more regularly oriented bundles of fibrils than the papillary dermis.
In the papillary dermis of healthy skin, dermal papillae are present at the interface between the dermis, epidermis, and basement membrane zone. These are finger-like projections of the papillary dermis into the epidermis. There is also a high concentration of fibroblasts in the papillary dermis, which proliferate rapidly, have increased metabolic activity, and synthesize proteoglycans—distinct from those found in the reticular dermis.
Fibroblasts are the primary cell types of dermis and they synthesize the extracellular structural proteins, such as collagen and elastin as well as glycosaminoglycans (e.g., hyaluronic acid), which are the major water holding components of the dermis. Together, these components constitute the extracellular matrix. In the sections below, we examine the major constituents of the dermis (collagen, elastin, and extracellular matrix) in greater detail. Figure 1 provides an illustration of one of the paramount functions of fibroblast cells, the synthesis of collagen. In the transmission electron micrograph, one may observe the strong association between a single fibroblast and neighboring synthesized collagen. It should be noted that depending on their location (superficial vs. deeper dermis), fibroblasts are known to show considerable functional heterogeneity.1
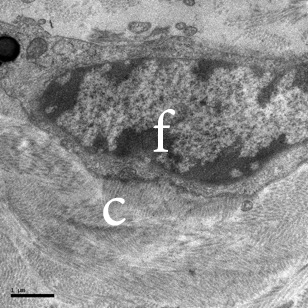
Figure 1: TEM image of a fibroblast (f) in human dermis. Note the intimate association of the fibroblast with the collagen fibers (C).
In addition to fibroblasts, the dermis is home to other cells, which are essential for immune system function. These immune cells consist of macrophages, mast cells, and other blood-borne cells, such as lymphocytes.
It is important to keep in mind that the dermis is not merely a structural foundation. Its components have an extremely significant biological role in skin health and disease.2 Ongoing research continues to explore and define the physical, chemical, and biological roles of various components of the dermis.
Collagen
As the most abundant protein in the body, especially in the dermis, collagen has attracted attention from researchers in the personal care industry for many years now. Collagen represents about 75% of the dry weight of the dermis and provides the skin with its strength and elasticity. It is a fibrous structural protein with a primary structure conforming to Gly-X-Y, where X=proline and Y=hydroxyproline. A single polypeptide chain of collagen exists in the alpha chain conformation (not to be confused with alpha helix). Three alpha chains are actually supertwisted together to form a triple helix (see Figure 2).
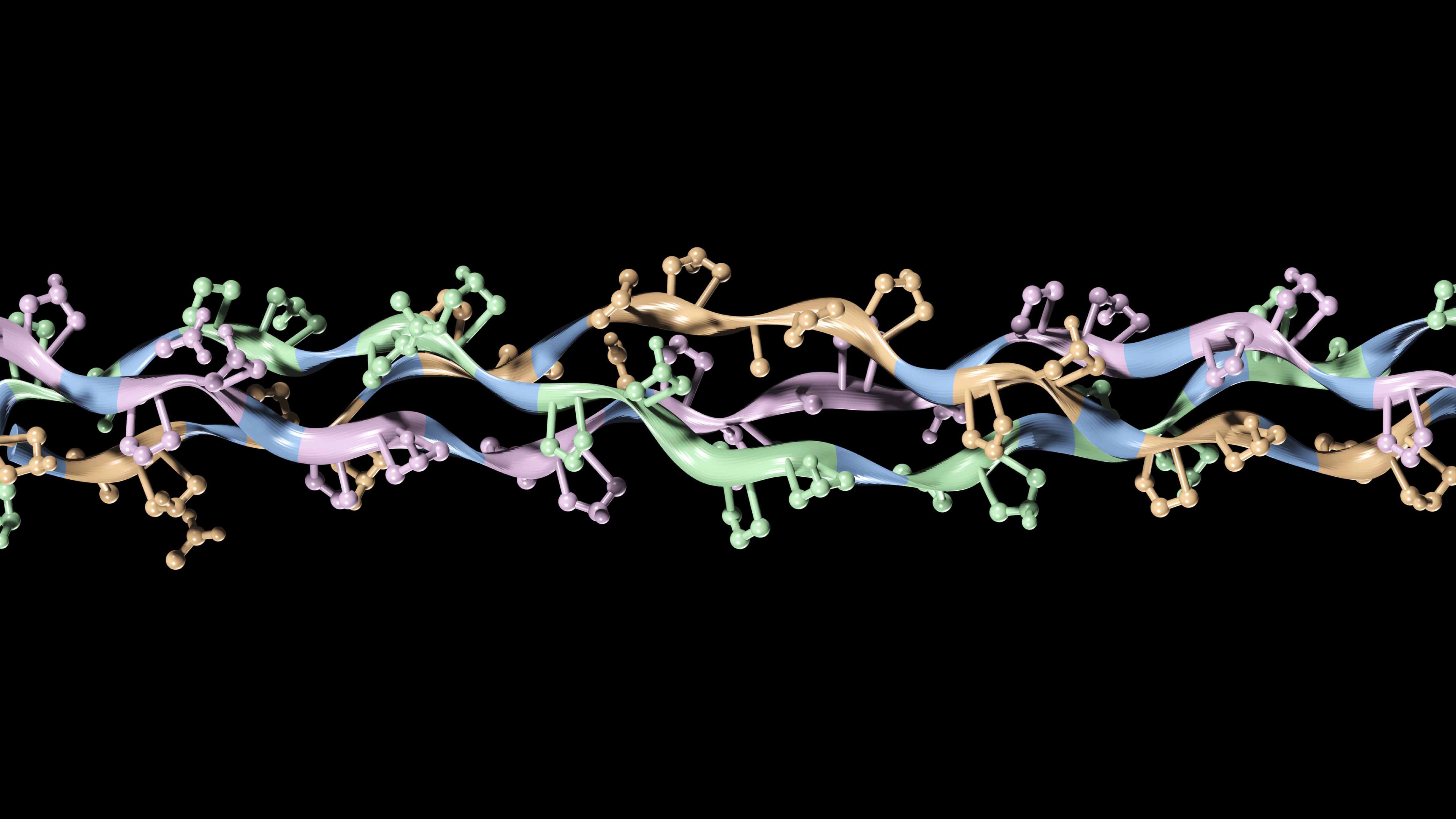
Figure 2: Tertiary structure of collagen. The alpha chains are supertwisted to form a triple helix.
The collagen fibers that we are so accustomed to seeing in scanning electron microscope (SEM) images are constructed from a number of triple helices. In Figure 3, an illustration is provided demonstrating the architecture of a collagen fiber formed by many triple helices that are crosslinked together (dark red bridge). A number of crosslinks exist in collagen, most notably those formed by pyridinoline and glycol-aldehyde pyridine. The bands that appear in the collagen fibers, as evident from the SEM micrographs, are the result of the arrangement of the collagen triple helices. The cross striations in the collagen fibers are due to spacing between the heads and tails of adjacent triple helices.
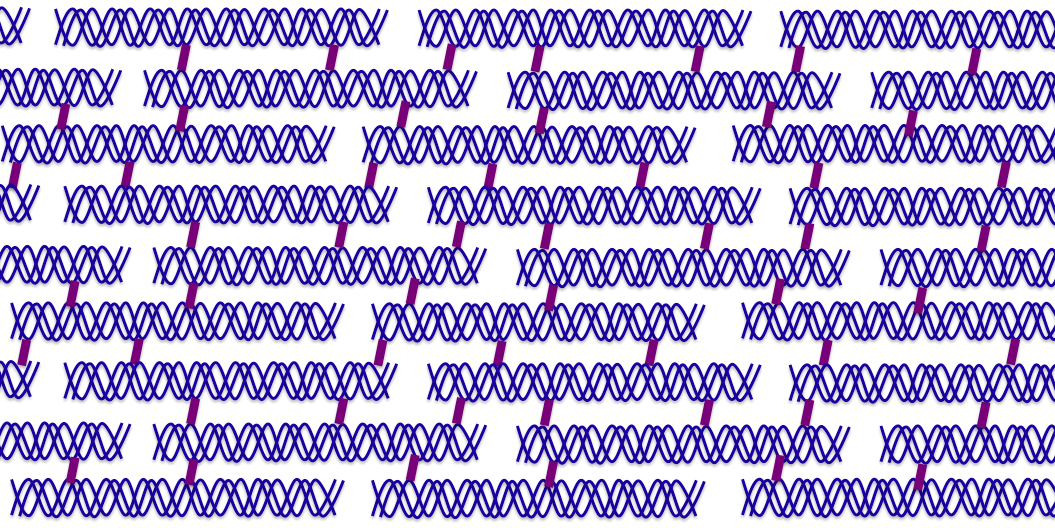
Figure 3: Illustration of the structure of a collagen fiber. Three alpha chains (blue) wrap around each other to form a triple helix. The supertwisted chains are crosslinked (dark red links) together to form a molecular hierarchy.
There are a number of different types of collagen, which differ from each other in terms of the polypeptide sequence and the hierarchal arrangement of triple helices. As we mentioned in the Basement Membrane Zone section, type VII collagen is predominantly found in the basement membrane. In addition, skin contains collagen types I, III, and V. There is roughly 80-90% type I collagen, 8-12% type III collagen, and <5% type V collagen. While type V collagen is a minor component of the total collagen content, it is very important for collagen fibrillogenesis. Type III collagen is more abundant in the papillary dermis, while the reticular dermis predominantly contains type I collagen.
Also associated with the dermis is a family of secreted proteoglycans that include decorin, biglycan, fibromodulin, lumican, epiphycan, and keratocan. Mutations in these proteins can lead to defective fibrillogenesis and adversely affect other functions of collagen as well. We discuss some properties of proteoglycans in one of the sections below.
Collagen crosslinking is achieved via the enzyme lysyl oxidase. Reduced activity of the enzyme can lead to collagen defects, such as that found in cutis laxa.3 This is a relatively rare disease in which the skin loses elasticity and hangs loosely. In general, defects in collagen lead to skin fragility and hyperelasticity. A good example is Ehlers-Danlos syndrome in which type III collagen levels are low. At the other end of spectrum are diseases like scleroderma where there is a highly abnormal level of collagen production. Physically, it is characterized by a hardening of the skin.
Elastic fibers
Compared to collagen, elastic fibers are much less abundant in the skin, only accounting for about 4% of the dry weight of the dermis. However, elastic fibers are critical for the resiliency of the skin, ensuring that stretched or deformed skin returns to its original position with a snap. Elastic fibers are also present in blood vessels and lymphatic tissue as well as the sheaths of hair follicles.
Elastic fibers exist as both microfibrillar and amorphous components in the dermis. The most prevalent and commonly known microfibrillar protein is fibrillin, but there are also others such as emilin, fibulin, and latent transforming growth factor-β-binding protein.4 The amorphous component of elastic fibers is elastin, which is comprised of tropoelastin crosslinked together to form the overall elastin molecular unit (see Figure 4).
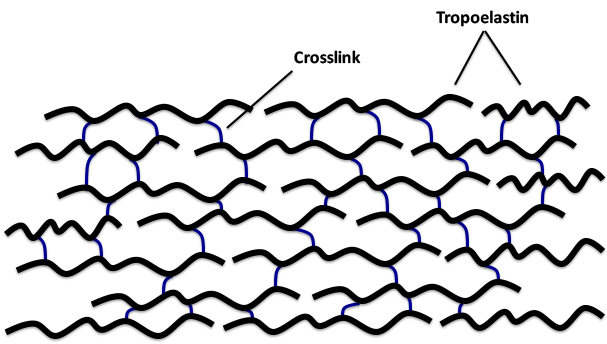
Figure 4: Illustration of the molecular architecture of elastin, which is formed by the crosslinking (blue links) of multiple units of tropoelastin (black chains).
Tropoelastin is a 70 KDa polypeptide with about one-third of its amino acid composition accounted for by glycine residues. It also contains a considerable amount of hydrophobic amino acids (e.g., alanine, valine) as well as proline. The crosslinks connecting the tropoelastin polypeptides are desmosine and isodesomosine (see Figure 5).
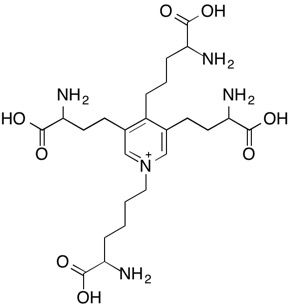
(a)
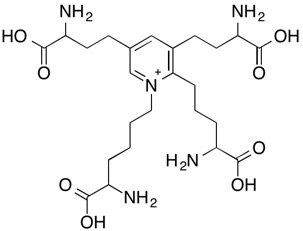
(b)
Figure 5: Molecular structures of crosslinks found in elastin: (a) desmosine and (b) isodesmosine.
Based on histology, elastic fibers of human skin are divided in to three types: oxytalan, elaunin, and elastic. This nomenclature is rather confusing since we collectively refer to all of the fibers as elastic fibers. Nevertheless, oxytalan fibers are primarily located in the papillary dermis. They are very thin fibers that are oriented perpendicular to the basement membrane zone and are formed by bundles of tubular microfibrils. Oxytalan fibers are connected to elaunin fibers, which in turn are linked to thicker elastic fibers in the reticular dermis that are oriented in a parallel fashion relative to the basement membrane zone.
The thicker elastic fibers of the reticular dermis contain significant amounts of the amorphous material, elastin, which is surrounded by microfibrils, while elaunin fibers contain microfibrils and only relatively small amounts of elastin.5 To reiterate, oxytalan fibers do not contain elastin—only microfibrils.
Microfibrils are constituents of elaunin, oxytalan, and elastic fibers that impart mechanical stability as well as elasticity to tissues. Their core is made of the glycoprotein fibrillin (as well as other microfibrillar proteins), microfibril associated proteins (MFAPs) and microfibril associated glycoproteins (MAGPs), which link microfibrils to elastin as well as to other extracellular matrix components and cells.
Elastic fibers are mostly developed in the infant years and only small amounts are made during the progressive aging of humans. Matrix metalloproteins (MMPs) are degradative enzymes synthesized by fibroblasts that destroy elastin structure. In photoageing, large-scale changes appear in the elastic fibers, referred to as elastosis, which results in a highly altered appearance and function.6
Dermal elastic fibers are also very important for the development and stabilization of the hair follicle. These elastic fibers are derived from cells lining the follicle and the arector pili muscle.7
Extracellular Matrix
The extracellular matrix of the dermis refers to a large component of the dermal compartment and consists of a network of macromolecules that reside in the extracellular areas not occupied by dermal cells. Collagen and elastin, already discussed above, are major components of the extracellular matrix in skin. In addition, other structural proteins of the extracellular matrix consist of dermatopontin, fibronectin, fibulins, fibrillins, laminins, nidogens, and tenascins.8,9 Further, there are a number of other components of the extracellular matrix, which include blood vessels and the cutaneous nerve network. [For the sake of brevity, we do not discuss the vascular and nerve components in this section.] Another important component of the extracellular matrix are the proteoglycans. They are glycoproteins that contain a core protein component that is covalently attached to a carbohydrate component, known as glycosaminoglycans (often referred to as GAGs). Extracellular matrix proteins play key roles in health and disease. Their functional implications include, but are not limited to, fibrillogenesis, wound healing, dermal remodeling, tumor promotion, angiogenesis, scarring, etc. In the paragraphs below we discuss some of the properties of several important extracellular matrix proteins.
Fibronectin
Fibronectin, an extracellular matrix protein, exists in its fibrillar form in the dermis and plays a significant role in cell migration and wound healing. It is a two subunit glycoprotein that can bind simultaneously to cell surface receptors, collagen, proteoglycans, and other types of molecules. Through its binding mechanism it provides connections between various components of the dermis and helps guide cells during processes that require cell movement (e.g., embryogenesis and wound healing).
Tenascins
Tenascins are a family of extracellular matrix proteins that appeared early in the evolution of vertebrates. There are four family members: tenascin-X, tenascin-R, tenascin-W, and tenascin-C. Tenascin-X associates with type I collagen, and its absence can cause Ehlers-Danlos Syndrome. The expression of tenascin-C and tenascin-W is developmentally regulated, and both are associated with cancer stroma and tumor blood vessels. Expression of tenascin-C is induced by infections and inflammation. [Tenascin-C knockout mice show a reduced inflammatory response.]
Dermatopontin
Dermatopontin is another evolutionally conserved protein that plays multiple roles in fibrillogenesis, re-epithelialization, and wound healing. By virtue of its role in modifying TGF-beta 1 activity, it also influences scar formation; dermatopontin is often noticeably absent in hypertrophic scars.10
Proteoglycans
The glycosaminoglycans are commonly referred to as the ground substance due to their histological appearance. They represent a fraction of a percent (~0.2%) of the dry weight of the dermis and, essentially, surround and embed the fibrous components of the dermis. The glycosaminoglycans are known for their ability to bind water, and are able to bind up to 1,000 times their volume in water. Interestingly, glycoproteins are very anionic in character and actually have the highest charge density of any macromolecules found in vertebrates.11 The high charge density property results in an extended chain conformation structure, leading to a viscous medium.
Figure 6 provides an illustration of a typical proteoglycan molecule. It consists of a hyaluronic acid backbone with pendant polypeptide chains. Covalently attached to each polypeptide chain are the glycosaminoglycan structures. They are polysaccharides that consist of repeating disaccharide units. The most common glycosaminoglycans are hyaluronic acid, dermatan sulfate, chondroitin sulfate, heparin, heparan sulfate, and keratan sulfate.
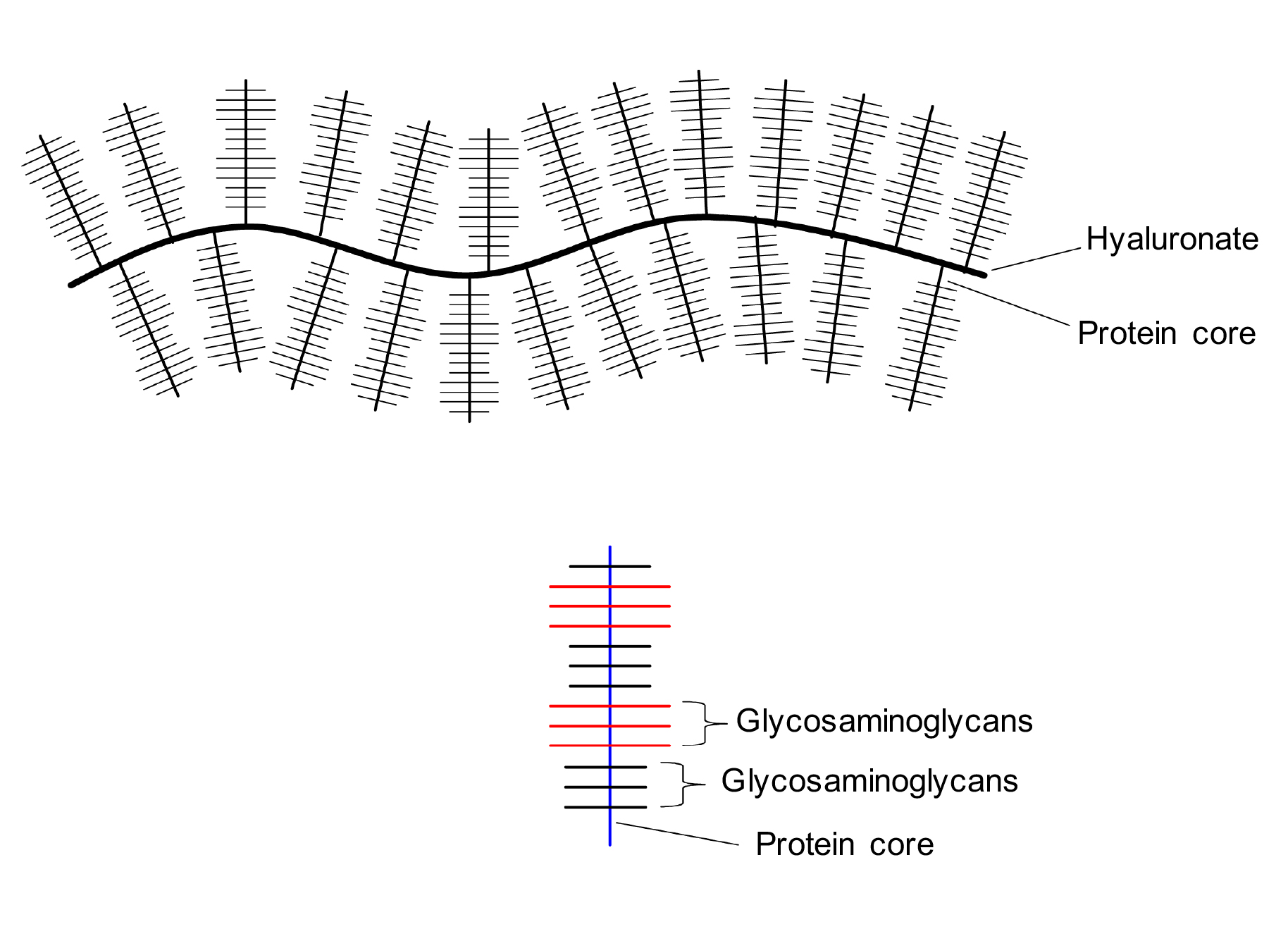
Figure 6: Illustration of proteoglycan structure. It is a large macromolecule that consists of a hyaluronic acid (hyaluronate) backbone with pendant protein chains (protein core). Attached to the proteins are glycosaminoglycans, which are polysaccharides containing repeating units of disaccharides.
References
1. J.M. Sorrell and A.I. Caplan, Fibroblast heterogeneity: more than skin deep, J. Cell Sci., 117, 667-675 (2004).
2. E. Gustafsson and R. Fässler, Insights into extracellular matrix functions from mutant mouse models, Exp. Cell Res., 261, 52-68 (2000).
3. J. Myllyharju and K.I. Kivirikko, Collagens, modifying enzymes and their mutations in humans, flies and worms, Trends Genet., 20, 33-43 (2004).
4. J. Uitto and M.-L. Chu, “Elastic fibers” in Fitzpatrick’s Dermatology in General Medicine, Vol. I, 6th ed., Eds. I.M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, and S.I. Katz, McGraw-Hill: New York (2003).
5. G. Cotta-Pereira, F. Guerra Rodrigo, S. Bittencourt-Sampaio,
Oxytalan, elaunin, and elastic fibers in the human skin, J. Invest. Dermatol., 66, 143-148 (1976).
6. J. Uitto, “Elastic fibers” in Biology of the Integument 2: Vertebrates, Eds. J. Bereiter-Hahn, A.G. Matoltsy, and K.S. Richards, Springer-Verlag: Berlin, 810-814 (1986).
7. B. Starcher, R.L. Aycock, and C.H. Hill, Multiple roles for elastic fibers in the skin, J. Histochem. Cytochem., 53, 431-443; doi: 10.1369/jhc.4A6484.2005 (2005).
8. T. Krieg and M. Aumailley, The extracellular matrix of the dermis: flexible structures with dynamic functions, Exp. Dermatol., 20, 689–695 (2011). doi:10.1111/j.1600-0625.2011.01313.x.
9. O. Okamoto and S. Fujiwara, Dermatopontin, a novel player in the biology of the extracellular matrix, Connect Tissue Res., 47, 177-189 (2006).
10. V.R. Krishnaswamy and P.S. Korrapati, Role of dermatopontin in re-epithelialization: implications on keratinocyte migration and proliferation, Sci. Rep.; Dec 9; 4, 7385 (2014); doi: 10.1038/srep07385.
11. R.L Gallo and J.M. Trowbridge, “Proteoglycans and glycosaminoglycans of skin” in Fitzpatrick’s Dermatology in General Medicine, Vol. I, 6th ed., Eds. I.M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, and S.I. Katz, McGraw-Hill: New York (2003).
RETURN TO STRUCTURE OF SKIN MENU