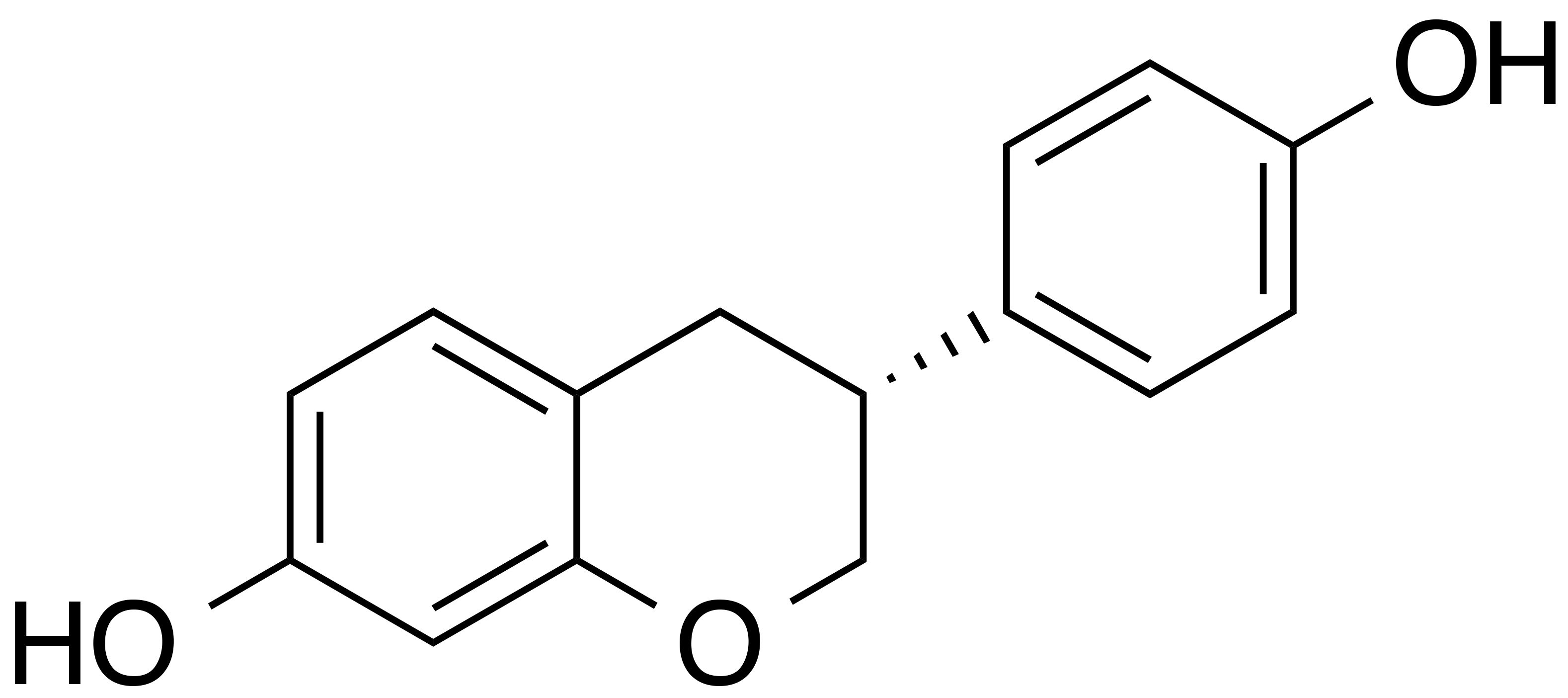
INCI name: N/A
Synonyms: 4’,7-isoflavandiol; 4’,7-dihydroxyisoflavan
Molecular formula: C15H14O3
Molecular weight: 242.3 g/mol
IUPAC name: (3S)-3-(4-hydroxyphenyl)-7-chromanol
CAS number: 531-95-3
EC number: 208-522-2
Equol is an isoflavanoid that has antioxidant, extracellular matrix enhancing, and phytoestrogenic properties.1 A considerable amount of research has gone into understanding its anti-aging properties in human skin.2,3 Equol is a metabolic product of daidzein—an isoflavone found in soybeans—which is metabolized by intestinal bacteria. Equol is also found in beans, cabbage, lettuce, and other plant species.
It should be noted that isoflavanoids are often referred to as phytoestrogens—meaning that they can exert biological effects by interacting with estrogen receptors. Interestingly, equol interacts with estrogen receptor beta, found in keratinocytes and fibroblasts, although the resulting biological outcome has not yet been elucidated.4 It also binds with 5 alpha-dihydrotestosterone thereby modulating androgenic activity, which reportedly inhibits prostate growth and also helps to prevent hair loss.5,6 Nevertheless, equol has led to the launch of several hair growth products in the market place.
In addition to its antioxidant and chemical messenger properties, there is a plethora of research demonstrating equol’s ability to stimulate the production of collagen, elastin, and TIMP (tissue inhibitor of metalloproteinases), thereby providing evidence (on the molecular scale) of its purported antiaging properties.1 Equol also stimulates the production of Nrf2—a transcription factor that is key in the regulation of the synthesis of antioxidant proteins.7
In addition, there is evidence that equol inhibits the activity of matrixmetalloproteinases and elastase—enzymes responsible for the degradation of extracellularmatrix proteins of the skin.2 Furthermore, there are various other proteins and enzymes that can be modulated by treatment with equol, providing evidence for the inhibition of inflammation and reduction in reactive oxygen species. However, it should be noted that the majority of these investigations have been conducted in vitro, utilizing cell culture systems, where researchers examined the modulation of various molecular markers. From a skin care perspective, future research in this area should focus on the clinical efficacy of this molecule.
Properties
Melting point: 192 – 193 °C8
[α]D = –23.5° (in ethanol)8
Solubility: ethanol and other organic solvents
References
1. E.D. Lephart, Skin aging and oxidative stress: equol’s anti-aging effects via biochemical and molecular mechanisms, Ageing Res. Rev., 31, 36-54 (2016).
2. R. Gopaul, H.E. Knaggs, and E.D. Lephart, Biochemical investigation and gene analysis of equol: a plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications, Biofactors, 38, 44-52 (2012).
3. R.L. Jackson, J.S. Greiwe, and R.J. Schwen, “S-equol, an antioxidant metabolite of soy daidzein, and oxidative stress in aging: a focus on skin and on the cardiovascular system” In Aging: Oxidative Stress and Dietary Antioxidants, Ed. V.R. Preedy, Elsevier: Amsterdam, 2014.
4. K.D. Setchell, C. Clerici, E.D. Lephart, S.J. Cole, C. Heenan, D. Castellani, B.E. Wolfe, L. Nechemias-Zimmer, N.M. Brown, T.D. Lund, R.J. Handa, and J.E. Heubi, S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora, Am. J. Clin. Nutr., 81, 1072-1079 (2005).
5. T.D. Lund, D.J. Munson, M.E. Haldy, K.D. Setchell, E.D. Lephart, and R.J. Handa, Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback, Biol. Reprod., 70, 1188-1195 (2004).
6. Soy ‘stops cancer and baldness’, BBC News, April 12, 2004; http://news.bbc.co.uk/2/hi/health/3607815.stm.
7. T. Zhang, X. Liang, L. Shi, L. Wang, J. Chen, C. Kang, J. Zhu, and M. Mi, Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells, PLoS One, 8(11), e79075 (2013). doi: 10.1371/journal.pone.0079075. eCollection 2013.
8. M.J. O’Neil, The Merck Index, 15th ed., The Royal Society of Chemistry: Cambridge, UK (2013).