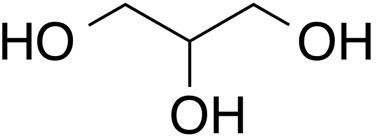
INCI name: glycerin
Synonyms: glycerin, glycerol
Molecular formula: C3H8O3
Molecular weight: 92.09 g/mol
IUPAC name: propane-1,2,3-triol
CAS number: 56-81-5
As one of the most ubiquitous chemicals in cosmetic products, glycerine has a long and rich history. There has been great interest in the cosmetic industry for its use in a variety of products, especially as a humectant in skin moisturizers.1 As noted by its structure, glycerine is characterized by its three hydroxyl groups, which in terms of chemical reactivity, two are equivalent. Glycerine is produced from natural sources (natural oils and fats) or synthetically (petrochemicals or carbohydrates). In fats and oils, glycerine is derived from the backbone of tryglycerides that have been saponified, hydrolyzed, or transesterified.2
Glycerine is a viscous colorless liquid that has a syrup-like consistency. It is non-toxic and characterized by its sweet taste. It is soluble in water and participates in hydrogen bonding by way of its three hydroxyl groups. In fact, this molecular characteristic gives glycerine its humectant properties in which it binds water from the atmosphere and maintains it in the skin.
Properties2
Melting point: 18.17 °C
Viscosity: 1499 cps (20 °C)
Refractive index: (Nd20) 1.47399
Freezing point (eutectic): (66.7% glycerol solution) – 46.5 °C
Log P (exp): -1.763
References
1. E. Jungermann and N.O.V. Sonntag, Glycerine: A Key Cosmetic Ingredient, Marcel Dekker: New York (1991).
2. The Soap and Detergent Association, Glycerine: An Overview, New York (1990).
3. S. Damodaran, K.L. Parkin, and O.R. Fennema, Fennema’s Food Chemistry, 4th ed., CRC Press: Boca Raton, FL (2007).