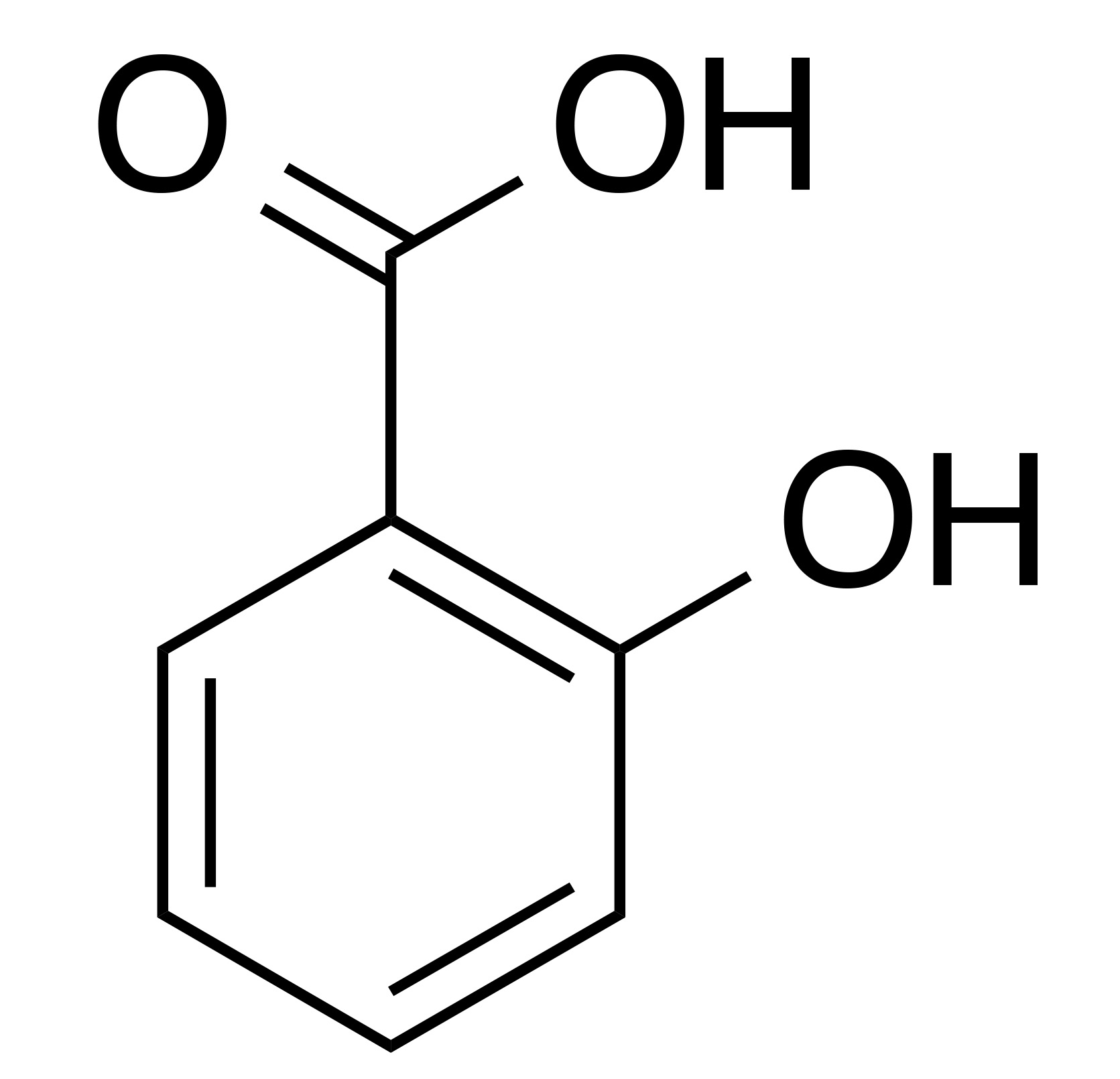
INCI name: salicylic acid
Synonyms: 2-hydroxybenzoic acid, ortho-hydroxybenzoic Acid
Molecular formula: C7H6O3
Molecular weight: 138.12 g/mol
IUPAC name: 2-hydroxybenzoic acid
CAS number: 69-72-7
EC number: 200-712-3
Salicylic acid belongs to a group of phenolic compounds found in plants. It is a plant hormone that has been investigated for its medicinal use in humans for over two hundred years.1 In plants, salicylic acid plays an important role in plant growth, uptake of ions, and photosynthesis.2 Since antiquity the bark and leaves of the willow tree were used as pain relievers in many parts of the world. It was not until the middle 19th century that researchers were able to identify the active ingredients from the tree as salicylic acid derivatives. Eventually, this led to attempts to synthesize salicylic acid on a large scale; however, the bitter taste and side effects (stomach inflammation) hindered its acceptance as an oral medication.2
In attempt to find a suitable alternative to salicylic acid, Felix Hoffman of Bayer pharmaceutical company synthesized acetylsalicylic acid (aspirin), which resulted in a molecule with less side effects during oral administration. Aspirin—a synthetic structural analog of salicylic acid—is used to treat a variety of ailments. It was introduced to the marketplace by Bayer in the late 1800s and has become one of the most popular medications worldwide.
Today, salicylic acid is used to treat a number of skin conditions including acne, hyperkeratotic disorders (e.g., psoriasis, ichthyoses, etc.), warts, and corns.3-5 It is a beta hydroxy acid that is used as a chemical peeling agent.5 Salicylic acid is a lipid soluble compound with desmolytic properties in which it breaks the junctions between corneocyte cells, thereby leading to weakened intercellular cohesion, ultimately resulting in exfoliation. It is by this mechanism that salicylic acid is so effective against many of the skin ailments discussed above. In addition, salicylic acid has antifungal properties and produces anesthetic effects. It decreases sebum production and also has comedolytic properties allowing it to function as an effective acne treatment.5
Properties
Density: 1.443 g/mL6
Boiling point: 211 °C6
Melting point: 159 °C6
LogP: log Kow = 2.188 ± 0.05486
pKa: 2.986
Refractive index: 1.5657
Solubility: water 0.20 (20 °C), 2.21 (80 °C); ethanol 34.87 (21 °C); soluble in boiling water6
References
1. A.C. Vlot, D.A. Dempsey, and D.F. Klessig, Salicylic acid a multifaceted hormone to combat disease, Annu. Rev. Phytopathol., 47, 177-206 (2009).
2. L. Popova, T. Pancheva, and A. Uzunova, Salicylic acid: properties, biosynthesis and physiological role, Bulg. J. Plant Physiol., 23, 85-93 (1997).
3. R.K. Madan and J. Levitt, A review of toxicity from topical salicylic acid preparations, J. Am. Acad. Dermatol., 70, 788-792 (2014).
4. M. Lebwohl, The role of salicylic acid in the treatment of psoriasis, Int. J. Dermatol., 38, 16-24 (1999).
5. T. Arif, Salicylic acid as a peeling agent: a comprehensive review, Clin. Cosmet. Invest. Dermatol., 8, 455-461 (2015).
6. M.J. O’Neil, The Merck Index, 15th ed., The Royal Society of Chemistry: Cambridge, UK (2013).
7. W.M. Haynes, Handbook of Chemistry and Physics, 97th ed., CRC Press: Boca Raton, FL (2016).